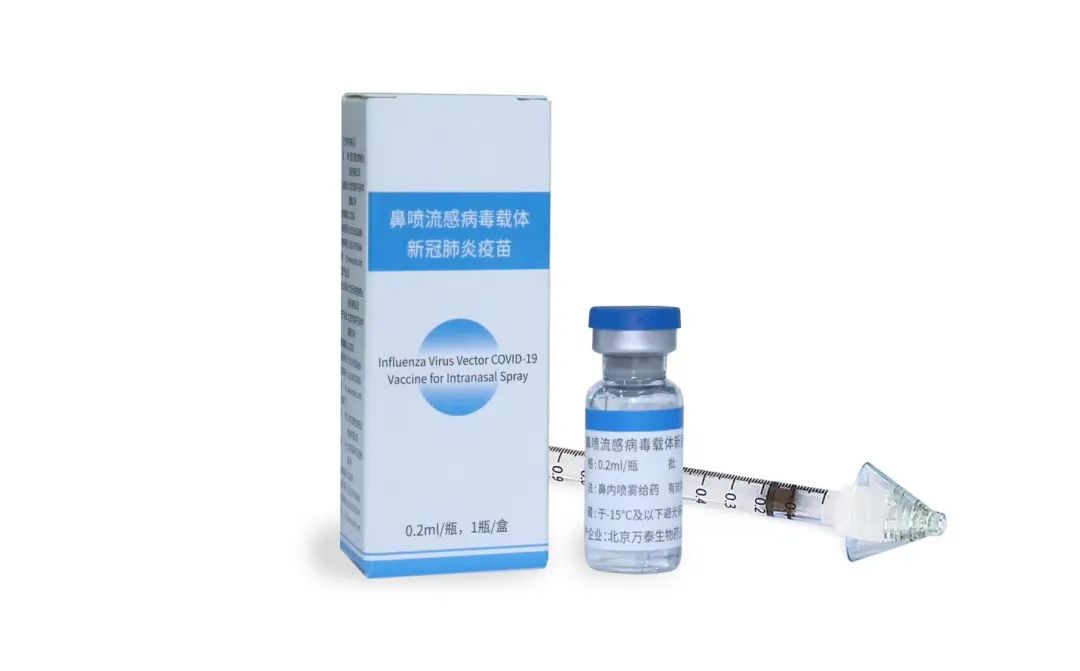
According to the press release of Xiamen University dated December 5, the Influenza Virus Vector Covid-19 Vaccine for Intranasal Spray (hereafter referred to as "Intranasal Spray Vaccine"), which was jointly developed by Xiamen University, University of Hong Kong, and Wantai BioPharm, was approved for urgent use. As a nasal spray, the Vaccine is the world's first intranasal spray COVID-19 vaccine that passed the phase III clinical trial, and is as effective and safe for the elderly as those for the young. The advantages of the Intranasal Spray Vaccine lie in that its immune barrier can effectively activate the immune protection mechanism of the body from the nasal cavity to the lung by imitating the way of virus infection. This mechanism is basically not affected by the virus' antibody escape mutation. The strength of the protective immune response to the prototype strain or to the main mutant strains so far including Omicron BF.7, XBB, and BQ.1.1 is equivalent.