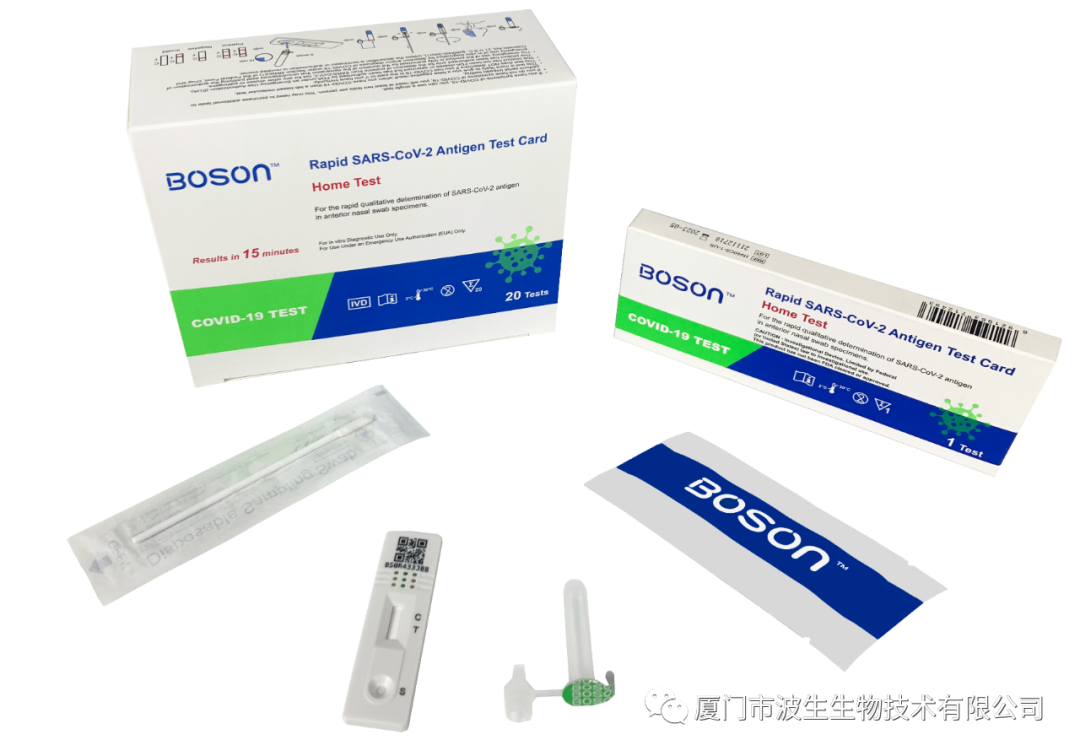
Recently, the COVID-19 antigen test kits manufactured by Xiamen Boson Biotech Co., Ltd. have been approved for emergency use authorization (EUA) by the Food and Drug Administration (FDA) of America, marking it the only Fujian biomedical enterprise receiving the EUA approval. After the occurrence of the COVID-19 epidemic, Boson Biotech rapidly developed COVID-19 fast test kits and obtained the CE certification. They are also "whitelisted" by the Ministry of Commerce of China and extensively exported to Asian, European, and American countries. In the first quarter of this year, Boson Biotech realized an operating income of RMB 1.8 billion, accounting for 73.2% of the full revenue of last year. In addition, other Xiamen-based listed biomedical enterprises also witnessed a significant profit growth last year, including AmoyDx, Double Medical, and Amoytop Biotech.