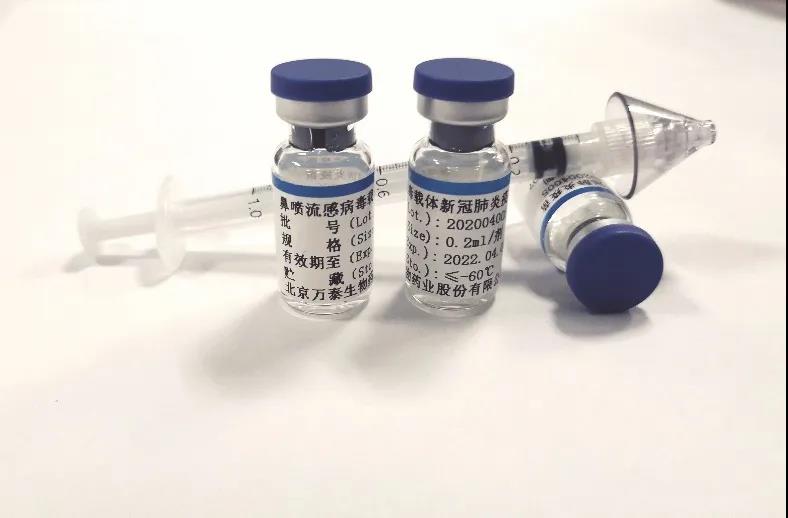
The COVID-19 vaccine now enables the vaccination by spraying into the nasal cavity rather than an injection. The nasal spray influenza virus vector COVID-19 vaccine, jointly developed by Xiamen University, the University of Hong Kong and Wantai BioPharm, has recently been approved for clinical trials with the emergency approval of the National Medical Products Administration. This also indicates that the COVID-19 vaccines that China has raced to develop by the five technical approaches have all been advanced to the clinical trial stage.
This is the only COVID-19 vaccine candidate approved for clinical trials that adopts nasal spray vaccination. The principle is that it exerts the protective effect by activating local immune response and systemic immune response through simulating the natural infection pathway of respiratory virus. One of its major benefits is that it is expected to prevent people from developing excessive immune responses when they are vaccinated.